Reducing the risk of contamination when working in a sterile environment is a demanding process. If the filling process cannot be controlled to prevent contamination, then the manufactured product poses a risk to patient safety and the entire batch of drug product is unusable.
Isolator-based filling lines and no-touch-transfer (NTT) systems are viable solutions to mitigating contamination risk, but each has weaknesses.
Pulsed light technology is a new sterilization solution in pharma manufacturing that not only further reduces the risk of contamination but also has many added benefits beyond powerful sanitation. In a Q&A with Lindsey Lungren, QC Microbiology Manager at Berkshire Sterile Manufacturing, and Dr. Christophe Riedel, CEO and founder of Claranor, we discuss how the technology works, the benefits in using it over other sanitation tools and its proven effectiveness against harmful bacteria and viruses.
What is pulsed light decontamination?
Pulsed light technology is an incredibly powerful sanitization tool which has existed for around 50 years, yet it is less well known than other sterilization technologies. Not only is it an extremely effective sterilant, but it is an eco-friendly, fast and safe option for sterilizing products. It also does not leave any residue on packaging, and it does not harm the items it sanitizes.

Many CMOs who specialize in small-scale fill-finish use NTT systems to prevent contamination from reaching isolated areas. This manual technique involves strategically debagging and removing a tub holding ready-to-use (RTU) containers from its packaging and transferring the tub to the next restricted access barrier system (RABS) or isolator. This allows the tubs with pre-sterilized containers, to pass into the isolator without being touched. If performed correctly, this system is very effective, but it still cannot eliminate the risk in which contaminates could be brought into the isolator on the tub’s surfaces. In a large filling line, with hundreds of tubs of RTU containers, the risk of contamination increases.
Why pulsed light?
Technologies such as flash steam sterilization, sterilant gases, and electron beam (e-beam) systems are potential solutions to these challenges but fall short in their own respects. Flash steam sterilization is not advanced enough to ensure the prevention of water from getting into the RTU tubs, using sterilant gases to sanitize primary containers prior to filling raises concern around product impact, and e-beam systems are large, costly and not environmentally friendly. Pulsed light technology is a powerful solution for effectively sterilizing incoming tubs with RTU containers which does not cause patient safety or product impact concerns, can handle accelerated line speeds and is safer and more sustainable than other options.

How does it work?
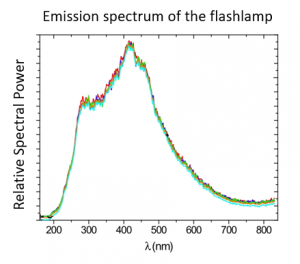
Pulsed light operates on the basic principle that microorganisms absorb energy. Each pulse lasts for 0.3 milliseconds and delivers 300 joules of energy. The lamp delivers 1 megawatt of power per pulse. Microorganisms will absorb light in the visible range if they have color (around 400 nm to 700 nm). They also absorb in the UVA-B range (280 nm – 400 nm). This absorption occurs without strong molecular change. However, microorganisms will also absorb energy in the UVC range (100 nm – 280 nm). This range of absorption causes dramatic molecular changes of photochemical effects, leading to covalent bond breakage which therefore destroys these microorganisms.
Why would it be preferred to no-touch-transfer systems?
Pulsed UV light is effective against bacteria and viruses, but it is also effective against harmful mold. In 2013, the photochemical and photothermal* effects of pulsed light on aspergillus mold, a common fungi that can cause disease in patients with weakened immune systems, were studied. This species absorbs at the UV, visible and IR wavelength ranges, but DNA and protein modification only occur in the presence of UVC light.
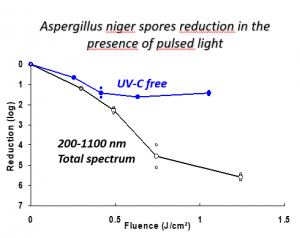
Wet chemical cleanings in closed restricted access barrier systems (RABS) may expect to reach a 4-log reduction in bioburden at best. In contrast, the study found that, in a single flash, a greater than 5-log reduction in more persistent, harder-to-kill spores was observed after a single pulse of light lasting less than a millisecond. Further studies of the light demonstrated that UVA, UVB and visible light all participate in the killing effect and that power plays a role in the sanitization process.
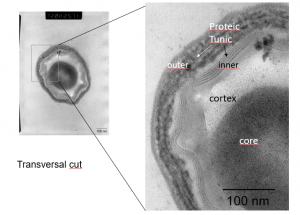
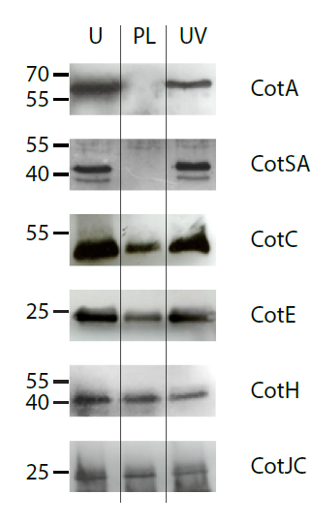
Another study looked at the pulsed light effect on subtilis spores. Subtilis spores have multiple layers of protective coats made up of 70 different proteins which make them especially difficult to destroy. When separate samples of these spores were treated with no light, with continuous UV light, and with pulsed light, results showed that only the pulsed light eliminated any of the proteins protecting the spore.
While NTT systems are effective when performed correctly, the addition of pulsed light decontamination improves sterility assurance by eliminating the reliance on operators to ensure sterility. NTT is used to pass already sterilized material into the filling line without contaminating the items in the process. The pulsed light decontamination chamber, offered by Steriline and Claranor, provides active decontamination of the tubs with RTU containers directly after they enter the isolator through NTT, and before their lid and liner are removed so filling can occur. This process eliminates any contamination that may have been brought into the isolator during ineffective NTT. As NTT systems typically involve operators to perform the action correctly, the tub decontamination system by pulsed light removes dependence on operators to ensure sterility is maintained.
Berkshire Sterile Manufacturing’s (BSM) sterility assurance
In order to create efficiency and sterility assurance in filling lines, BSM selected an isolator-based filling line to provide a much greater level of sterility assurance when compared to traditional cleanrooms and RABS. BSM will be the first company in the United States to incorporate pulsed light technology into a sterile filling line. The line is currently under construction and will be in operation in the first quarter of 2022. This technology will sanitize tubs containing RTU containers to a 6-log reduction in bioburden, significantly improving sterility assurance over the industry standard, no-touch-transfer systems.
Berkshire Sterile Manufacturing and Sharp have partnered to offer clients a seamless, end-to-end pharmaceutical manufacturing, packaging and distribution solution specifically aimed at small-batch syringes, vials and cartridges. Get in touch to learn how our combined expertise can work for you.
If you would like to learn more about our state-of-the-art filling line, get in touch.
*increasing the temperature in the microorganism until combustion
References:
G. Clair, et al., 2020. The spore coat is essential for Bacillus subtilis spore resistance to pulsed light, and pulsed light treatment eliminates some spore coat proteins. Int. J. Food Microbiol. 232, 108592.
Esbelin, J., Mallea, S., Ram, A. F., and Carlin, F. (2013). Role of pigmentation in protecting Aspergillus niger conidiospores against pulsed light radiation. Photochem. Photobiol. 89, 758–761.
